EISAI AND DeNA TO PROVIDE SMARTPHONE APP “EASIIT APP” THROUGH BUSINESS ALLIANCE AGREEMENT
EISAI DEMENTIA PLATFORM EASIIT COMMENCES
Eisai Co., Ltd. (Headquarters: Tokyo, CEO: Haruo Naito, “Eisai”) and DeNA Co., Ltd.’s subsidiary DeSC Healthcare Co., Ltd. (Headquarters: Tokyo, CEO: Sho Segawa, CMO: Kuniaki Miyake, “DeNA”) announced that they have begun provision of the brain performance application “Easiit” (non-medical device, referred to below as “Easiit App”), for preparation against dementia, on July 28, 2020. This provision is based on a business alliance agreement aiming for support and creation of new solutions in the dementia area as well as co-development of the Easiit App as a base element of the digital platform for dementia that Eisai is currently constructing. With the beginning of provision of the Easiit App, the Eisai Dementia Platform Easiit has commenced.
Eisai possesses over 35 years of experience in medicine creation and business activity in the dementia field, and DeNA has demonstrated performance in providing healthcare services and altering consumer behavior while applying know-how from its gaming and sports industry experiences with the theme of “Staying healthy with fun”. Both companies aim to contribute towards better health practices by all people through such approaches as visualizing brain and body health data, supporting brain performance maintenance, and providing useful lifestyle information. Both companies will continue their efforts in partnership for creating the digital platform, including the expansion of functions of the Easiit App.

Background on the co-development and provision of the Easiit App
In its medium-term business plan, EWAY2025, Eisai is aiming to become a “Medico Societal Innovator”, a company that changes society through creating medicines and providing various innovative solutions that change society. Particularly in the dementia field, Eisai is collaborating with partners such as medical organizations, diagnostic development companies, research organizations, and bio-ventures in addition to private insurance agencies, fitness clubs, automobile makers, retailers, and care facilities to realize construction of a “Dementia Ecosystem” for delivering new benefits. The base of this ecosystem will be the Dementia Platform Easiit. Through this platform, Eisai aims to collect information from participants, combine this information with Eisai’s independent data set comprising elements such as know-how, experience, and clinical data, and perform analysis in compliance with relevant regulations in order to deliver new benefits to participants in the form of various healthcare predictions and advice.
DeNA’s healthcare business aims for the extension of “healthy life expectancy” by realizing the conversion of “sick care” through treatment after becoming sick to “healthcare” through preventing sickness from occurring. DeNA provides various internet based healthcare services that apply its unique know-how for making enjoyable user experiences and extending use as cultivated through its activities in the gaming and sports fields.
In recent years, various research has demonstrated the possibility that decline in brain health may be mitigated through readjustments to lifestyle such as regular exercise, a well-balanced diet, and social interaction. On the other hand, according to a survey conducted by Eisai, the number of people who understand the correct preventive measures or perform cognitive function checks regularly are few, which indicates disparities (“chasms”).
The Easitt App, which aims to contribute to the promotion of healthy habits by making brain and body health visible, is core to Eisai’s digital platform business directed at eliminating these chasms. Both companies combined their respective strengths to co-develop this app, and have now begun provision of the first version.
The brain performance app “Easiit App”
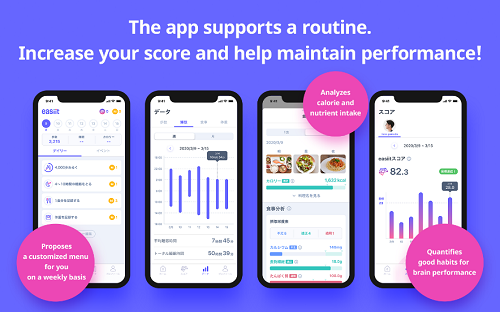
In the Easiit App, a menu of individualized recommendations based on users’ footsteps, diet, sleep, and weight records (lifelog) is updated on a weekly basis and displayed. Individualized scoring is conducted based on actions and habits which are good for brain performance. The Easiit App confirms score changes and breakdown, and encourages formation of good habits for brain performance moving forward. For diet record in particular, easy diet management is made possible as the Easiit App evaluates users’ meals via photo upload for calorie intake and eleven essential nutrients, and displays this information in relation to an age-based standard for calorie and nutrient intake. Additionally, with every use of the app one can collect Easiit miles, which can be exchanged for prizes such as gift cards. Through connection to wearables and subsequent functions such as sleep time tracking, the Easiit App can encourage the creation of good habits for brain performance.
All of the functions within the Easiit App are designed, developed, and operated on a framework for protection of individual information.
Planned functions moving forward
In the end of September of this year, Eisai plans to equip the Easiit App with a linkage to the brain performance self-check tool “NouKNOW” (non-medical device), which Eisai is currently selling to legal entities. In the future, the addition of a new function is planned for use in families in which members are living separately from each other.
Additionally, Eisai is investigating future equipment of the Easiit App with a function allowing for connection to medical data (as a non-medical device) on top of daily lifestyle data.
Commentary from persons in charge of operations in both companies
Eisai Vice President, President of Dementia Total Inclusive Ecosystem Business Unit and Chief Digital Officer Keisuke Naito said, “I believe that recording the health state of one’s brain and body alike and making those records visible is critical to the realization of one’s well-being in one’s own way. This app, which we have co-developed with DeNA, enables one to easily record one’s footsteps, sleep time, diet, nutrient deficiencies, and other elements. Through the expansion and wide adoption of the Easiit App, we will work steadfastly in this first step towards the realization of a society in which anyone may measure their own brain performance easily, make lifestyle improvements for the future, and receive early stage medical examination.”
DeNA Vice President, Head of Healthcare Business Division and President of DeSC Healthcare Co., Ltd. Sho Segawa said, “I encountered Eisai at a time when I was watching my parents care for my grandfather, who had developed dementia, and thinking to myself, ‘Is there not something I can do as a player of the healthcare industry?’. With the combination of Eisai’s experience in the dementia field and DeNA’s services, we will concentrate our expertise for encouraging and sustaining enjoyable healthy lifestyles in the Easiit App, making an effort to accelerate the creation of solutions for dementia.”
[Notes to editors]
1.?About brain performance (brain health) and issues surrounding it
In recent years, various research has demonstrated the possibility that decline in brain performance (brain health) may be mitigated through major readjustments to lifestyle such as regular exercise, a well-balanced diet, and social interaction. On the other hand, according to survey* conducted by Eisai on men and women in Japan between forty and seventy-nine years of age, it was revealed that 55.7% of participants understood the meaning and contents behind early assessment and prevention, 19.7% of participants were taking correct preventive actions in diet, exercise, sleep, etc. on a regular basis, and no more than 2.1% of participants were habitually performing self-assessments of cognitive function. These statistics represent disparities (“chasms”) which must be overcome in order to promote disease understanding and the incorporation of cognitive function checks into daily lifestyle.
*Independent online survey conducted in December 2019 by Eisai in Japan on participants in their 40’s, 50’s, 60’s, and above 70, with 200 male and 200 female participants per age bracket (total: 1,600).
2.?Summary of Easiit App details
Availability: iOS version available July 28, 2020 (Tuesday), Android version planned availability for end of August 2020.
Cost: Free of charge; the high function edition with various additional contents and continuous individual data visualization is to be launched for a charge in the winter of 2020.
Supervising editor: Dr.?Atsushi Iwata, Director, Department of Neurology, Tokyo Metropolitan Geriatric Hospital and Institute of Gerontology
Easiit website:?https://www.easiit.com/app
<Functions, properties>
- Presents weekly menu customized for the user, including suggestions for exercise, diet, sleep, etc.
- Presents footstep count, diet record, sleep time, and body weight in graph form for user confirmation
- Evaluates users’ meal photos for amount of calories and eleven essential nutrients and displays this information in relation to an age-based standard for intake
- Awards Easiit miles with every use, which can be collected and exchanged for gift cards, etc.
- Connects with the Fitbit wearable device to automate footstep count, sleep time, and body weight measurement
- Conducts individual scoring [Easiit Score] based on records of actions and habits which are good for brain performance in correspondence with the menu. The Easiit Score provides a positive motivation for the user to practice a lifestyle that is good for brain performance.
3. About the Dementia Ecosystem business
Leveraging experience gained from the development and marketing of Aricept?, a treatment for Alzheimer’s disease and dementia with Lewy bodies, Eisai aims to establish the “Dementia Platform Easiit” for analyzing participants’ health and lifestyle information and subsequently providing advice on brain health. In the future, Eisai is planning to evolve this platform to a digital platform that spans areas of daily life and medicine.
With the digital platform as a base, Eisai aims to construct a “Dementia Ecosystem” for delivering new benefits by collaborating with partners such as medical organizations, diagnostic development companies, research organizations, and bio-ventures in addition to private insurance agencies, finance, fitness clubs, automobile makers, retailers, and care facilities.
4. About Eisai Co., Ltd.
Eisai Co., Ltd. is a leading global pharmaceutical company headquartered in Japan. Eisai’s corporate philosophy is based on the?human health care?(hhc) concept, which is to give first thought to patients and their families, and to increase the benefits that health care provides to them. With a global network of R&D facilities, manufacturing sites and marketing subsidiaries, we strive to realize our?hhc?philosophy by delivering innovative products to target diseases with high unmet medical needs, with a particular focus in our strategic areas of Neurology and Oncology. For more information about Eisai Co., Ltd., please visit?https://www.eisai.com.
5. About DeNA’s healthcare initiatives
With its healthcare business mission of “Staying healthy with fun”, DeNA provides healthcare services such as its gene screening service “MYCODE” and its healthcare engagement app “kencom” aimed at healthcare insurance agencies and municipalities, applying engagement science as cultivated through its gaming and sports businesses. DeNA was selected in 2019 and 2020 consecutively for the “Health and Productivity Branding”, a designation that is granted by Japan’s Ministry of Economy, Trade, and Industry in collaboration with the Tokyo Stock Exchange for companies that are evaluated to think and act strategically with an operational perspective for the health of their employees.
6. About DeNA Co., Ltd.
DeNA Co., Ltd.’s corporate mission is to “Delight and Impact the World”. Applying its strengths in internet and AI, DeNA aims to create various solutions for various social issues in fields ranging from entertainment, including gaming and social concerts, to an expanding sports presence, including sponsorship of the Yokohama Bay Stars baseball team, as well as in the healthcare and automotive industries. For more information about DeNA Co., Ltd., please visit?https://dena.com/intl/.
Media Inquiries
Eisai Co., Ltd.
Public Relations Department
TEL : +81-(0)3-3817-5120
DeNA Co., Ltd.
PR Group
e-mail: pr@dena.jp

.jpg)