Eisai is a multinational company specializing in the research and development of pharmaceutical products, headquartered in Tokyo and founded in 1941. Its overseas branches, subsidiaries and factories are located in Europe, the Americas and Asia, with a total capitalization of 44,986 million yen and a global workforce of more than 10,000 employees. In FY2022, Eisai reached 744 billion yen (approximately RMB 38 billion) globally, with an investment of 173 billion yen (approximately RMB 8.8 billion) in research and development (R&D).
Since Eisai entered the Chinese market in the early 1990s, it has been developing smoothly, establishing Shenyang Eisai Pharmaceutical Co., Ltd. in 1991 and Eisai (Suzhou) Pharmaceutical Co., Ltd. in 1996, and then it officially renamed? to Eisai China Inc. in 2002. Along with the development of its business in China, now its member companies include Eisai China Holdings Ltd., Eisai China Inc., Eisai (Liaoning) Pharmaceutical Co., Ltd., Eisai (Suzhou) Trading Co., Ltd., Eisai (Hong Kong) Co., Ltd., and it established a joint-venture company “Unlimit Health Limited.”
At present, the total registered capital in China is US$108.54 million, and its HQ in China is located in Shanghai. Eisai China has formed a scale of development to sell dozens of pharmaceuticals in China, focusing on the fields of NTA, Oncology (Special Care), GI, etc., and expanding the field of generic drugs and Internet+medicine files. Eisai China is ranked 17th of global multinational pharmaceutical companies in China in 2022.
]]>LENVIMA? Plus Everolimus Also Showed Statistically Significant Improvement in PFS and ORR Endpoints Versus Sunitinib
Results of Investigational Phase 3 CLEAR Trial (Study 307)/KEYNOTE-581 to be Presented at Upcoming Medical Meeting
TOKYO and KENILWORTH, N.J., Nov. 10, 2020 – Eisai Co., Ltd. (Headquarters: Tokyo, CEO: Haruo Naito, “Eisai”) and Merck & Co., Inc., Kenilworth, N.J., U.S.A. (known as MSD outside the United States and Canada) today announced new investigational data demonstrating positive top-line results from the pivotal Phase 3 CLEAR trial (Study 307)/KEYNOTE-581 evaluating LENVIMA?, the orally available multiple receptor tyrosine kinase inhibitor discovered by Eisai, plus KEYTRUDA?, the anti-PD-1 therapy from Merck & Co., Inc., Kenilworth, N.J., U.S.A., as well as LENVIMA plus everolimus versus sunitinib for the first-line treatment of patients with advanced renal cell carcinoma (RCC).
LENVIMA plus KEYTRUDA met the trial’s primary endpoint of Progression-Free Survival (PFS) and its key secondary endpoints of Overall Survival (OS) and Objective Response Rate (ORR), demonstrating a statistically significant and clinically meaningful improvement in PFS, OS and ORR versus sunitinib in the intention-to-treat (ITT) study population. LENVIMA plus everolimus also met the trial’s primary endpoint of PFS and a key secondary endpoint of ORR, demonstrating a statistically significant and clinically meaningful improvement in PFS and ORR versus sunitinib in the ITT study population. The ITT population included patients across all Memorial Sloan Kettering Cancer Center (MSKCC) risk groups (favorable, intermediate and poor). The safety profiles of both LENVIMA plus KEYTRUDA and LENVIMA plus everolimus were consistent with previously reported studies. Eisai and Merck & Co., Inc., Kenilworth, N.J., U.S.A. will discuss these data with regulatory authorities worldwide, with the intent to submit marketing authorization applications based on these results, which will be presented at an upcoming medical meeting.
“The results for KEYTRUDA plus LENVIMA versus sunitinib, which showed a statistically significant improvement in progression-free survival, overall survival and objective response rate, build on the growing scientific evidence that supports the investigation of KEYTRUDA-based combinations for the first-line treatment of advanced renal cell carcinoma,” said Dr. Gregory Lubiniecki, Associate Vice President, Oncology Clinical Research, Merck Research Laboratories. “Merck and Eisai are committed to working together to continue to explore the potential of the KEYTRUDA plus LENVIMA combination, particularly in areas of great unmet need such as renal cell carcinoma.”
“The results from CLEAR (Study 307)/KEYNOTE-581 support the potential use of KEYTRUDA plus LENVIMA for the first-line treatment of advanced RCC. These data also support the potential first-line use of LENVIMA plus everolimus, which is already approved in advanced RCC following prior antiangiogenic therapy,” said Dr. Takashi Owa, Vice President, Chief Medicine Creation and Chief Discovery Officer, Oncology Business Group at Eisai. “These findings energize our efforts as we continue to advance our understanding and address the unmet needs of patients with difficult-to-treat cancers.”
Eisai and Merck & Co., Inc., Kenilworth, N.J., U.S.A. are continuing to study the LENVIMA plus KEYTRUDA combination through the LEAP (LEnvatinib And Pembrolizumab) clinical program across 19 trials in 13 different tumor types (endometrial carcinoma, hepatocellular carcinoma, melanoma, non-small cell lung cancer, RCC, squamous cell carcinoma of the head and neck, urothelial cancer, biliary tract cancer, colorectal cancer, gastric cancer, glioblastoma, ovarian cancer and triple-negative breast cancer).
?
About CLEAR (Study 307)/KEYNOTE-581
CLEAR (Study 307)/KEYNOTE-581 is a multi-center, randomized, open-label, Phase 3 trial (ClinicalTrials.gov,?NCT02811861) evaluating LENVIMA in combination with KEYTRUDA or in combination with everolimus versus sunitinib alone for the first-line treatment of patients with advanced RCC. The study enrolled approximately 1,050 patients who were randomized to one of three treatment arms to receive LENVIMA (18 mg orally once daily) in combination with everolimus (5 mg orally once daily) [arm A]; or LENVIMA (20 mg orally once daily) in combination with KEYTRUDA (200 mg intravenously every three weeks) [arm B]; or sunitinib (50 mg orally once daily for four weeks on treatment, followed by two weeks off treatment) [arm C]. The primary endpoint is comparison of PFS between arm A versus arm C, and arm B versus arm C, by independent review per RECIST v1.1 criteria. Key secondary endpoints include OS, ORR and safety.
About Renal Cell Carcinoma (RCC)
Worldwide, it is estimated there were more than 403,000 new cases of kidney cancer diagnosed and more than 175,000 deaths from the disease in 2018.1?In Japan, there were over 24,000 new cases and 8,000 deaths in 2018.1?In the U.S. alone, it is estimated there will be nearly 74,000 new cases of kidney cancer diagnosed and almost 15,000 deaths from the disease in 2020.?2?Renal cell carcinoma is by far the most common type of kidney cancer; about nine out of 10 kidney cancers are RCCs.3Renal cell carcinoma is about twice as common in men as in women.4?Most cases of RCC are discovered incidentally during imaging tests for other abdominal diseases. Approximately 30% of patients with RCC will have metastatic disease at diagnosis, and as many as 40% will develop metastases after primary surgical treatment for localized RCC.5,6?Survival is highly dependent on the stage at diagnosis, and with a five-year survival rate of 12% for metastatic disease, the prognosis for these patients is poor.7
?
About LENVIMA??(lenvatinib) Capsules
LENVIMA, discovered and developed by Eisai, is a kinase inhibitor that inhibits the kinase activities of vascular endothelial growth factor (VEGF) receptors VEGFR1 (FLT1), VEGFR2 (KDR), and VEGFR3 (FLT4). LENVIMA inhibits other kinases that have been implicated in pathogenic angiogenesis, tumor growth, and cancer progression in addition to their normal cellular functions, including fibroblast growth factor (FGF) receptors FGFR1-4, the platelet derived growth factor receptor alpha (PDGFRα), KIT, and RET. In syngeneic mouse tumor models, lenvatinib decreased tumor-associated macrophages, increased activated cytotoxic T cells, and demonstrated greater antitumor activity in combination with an anti-PD-1 monoclonal antibody compared to either treatment alone. The combination of LENVIMA and everolimus showed increased anti-angiogenic and anti-tumor activity as demonstrated by decreased human endothelial cell proliferation, tube formation, and VEGF signaling in vitro and tumor volume in mouse xenograft models of human renal cell cancer greater than each drug alone. Currently, LENVIMA has been approved for monotherapy as a treatment for thyroid cancer in over 65 countries including Japan, the United States, in Europe and Asia, and for unresectable hepatocellular carcinoma in over 65 countries including Japan, the United States, in Europe, China and in Asia. Additionally, it is also approved in combination with everolimus as a treatment for renal cell carcinoma following prior antiangiogenic therapy in over 55 countries, including the United States, in Europe and Asia. In Europe, the agent was launched under the brand name Kisplyx??for renal cell carcinoma. In addition, it is approved in combination with KEYTRUDA as a treatment for advanced endometrial cancer that is not microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR), who have disease progression following prior systemic therapy and are not candidates for curative surgery or radiation in countries including the United States, Australia and Canada. Continued approval for this indication is contingent upon verification and description of clinical benefit in the confirmatory trials.
?
About KEYTRUDA??(pembrolizumab) Injection
KEYTRUDA is an anti-PD-1 therapy that works by increasing the ability of the body’s immune system to help detect and fight tumor cells. KEYTRUDA is a humanized monoclonal antibody that blocks the interaction between PD-1 and its ligands, PD-L1 and PD-L2, thereby activating T lymphocytes which may affect both tumor cells and healthy cells.
Merck & Co., Inc., Kenilworth, N.J., U.S.A. has the industry’s largest immuno-oncology clinical research program. There are currently more than 1,200 trials studying KEYTRUDA across a wide variety of cancers and treatment settings. The KEYTRUDA clinical program seeks to understand the role of KEYTRUDA across cancers and the factors that may predict a patient’s likelihood of benefitting from treatment with KEYTRUDA, including exploring several different biomarkers.
?
About the Merck& Co., Inc., Kenilworth, N.J., U.S.A. and Eisai Strategic Collaboration
In March 2018, Eisai and Merck & Co., Inc., Kenilworth, N.J., U.S.A., known as MSD outside the United States and Canada, through an affiliate, entered into a strategic collaboration for the worldwide co-development and co-commercialization of LENVIMA. Under the agreement, the companies will jointly develop, manufacture and commercialize LENVIMA, both as monotherapy and in combination with KEYTRUDA, the anti-PD-1 therapy from Merck & Co., Inc., Kenilworth, N.J., U.S.A.
In addition to ongoing clinical studies evaluating the KEYTRUDA plus LENVIMA combination across several different tumor types, the companies have jointly initiated new clinical studies through the LEAP (LEnvatinib And Pembrolizumab) clinical program and are evaluating the combination in 13 different tumor types (endometrial carcinoma, hepatocellular carcinoma, melanoma, non-small cell lung cancer, renal cell carcinoma, squamous cell carcinoma of the head and neck, urothelial cancer, biliary tract cancer, colorectal cancer, gastric cancer, glioblastoma, ovarian cancer and triple-negative breast cancer) across 19 clinical trials.
?
Eisai’s Focus on Cancer
Eisai focuses on the development of anticancer drugs, targeting the tumor microenvironment (with experience and knowledge from existing in-house discovered compounds) and the driver gene mutation and aberrant splicing (leveraging RNA Splicing Platform) as areas (Ricchi) where real patient needs are still unmet, and where Eisai can aim to become a frontrunner in oncology. Eisai aspires to discover innovative new drugs with new targets and mechanisms of action from these?Ricchi, with the aim of contributing to the cure of cancers.
About Eisai
Eisai is a leading global research and development-based pharmaceutical company headquartered in Japan, with approximately 10,000 employees worldwide. We define our corporate mission as “giving first thought to patients and their families and to increasing the benefits health care provides,” which we call our?human health care(hhc) philosophy. We strive to realize our?hhc?philosophy by delivering innovative products in therapeutic areas with high unmet medical needs, including Oncology and Neurology. In the spirit of?hhc, we take that commitment even further by applying our scientific expertise, clinical capabilities and patient insights to discover and develop innovative solutions that help address society’s toughest unmet needs, including neglected tropical diseases and the Sustainable Development Goals.
For more information about Eisai, please visit?www.eisai.com?(for global),?us.eisai.com(for U.S.) or?www.eisai.eu?(for Europe, Middle East, Africa), and connect with us on Twitter (U.S.?and?global) and?LinkedIn?(for U.S.).
?
Merck?& Co., Inc., Kenilworth, N.J., U.S.A.’s Focus on Cancer
Our goal is to translate breakthrough science into innovative oncology medicines to help people with cancer worldwide. At Merck & Co., Inc., Kenilworth, N.J., U.S.A., the potential to bring new hope to people with cancer drives our purpose and supporting accessibility to our cancer medicines is our commitment. As part of our focus on cancer, Merck & Co., Inc., Kenilworth, N.J., U.S.A. is committed to exploring the potential of immuno-oncology with one of the largest development programs in the industry across more than 30 tumor types. We also continue to strengthen our portfolio through strategic acquisitions and are prioritizing the development of several promising oncology candidates with the potential to improve the treatment of advanced cancers. For more information about our oncology clinical trials, visit?www.merck.com/clinicaltrials.
About Merck?& Co., Inc., Kenilworth, N.J., U.S.A.?
For more than 125 years, Merck & Co., Inc., Kenilworth, N.J., U.S.A., known as MSD outside of the United States and Canada, has been inventing for life, bringing forward medicines and vaccines for many of the world’s most challenging diseases in pursuit of our mission to save and improve lives. We demonstrate our commitment to patients and population health by increasing access to health care through far-reaching policies, programs and partnerships. Today, Merck & Co., Inc., Kenilworth, N.J., U.S.A. continues to be at the forefront of research to prevent and treat diseases that threaten people and animals – including cancer, infectious diseases such as HIV and Ebola, and emerging animal diseases – as we aspire to be the premier research-intensive biopharmaceutical company in the world. For more information, visit?www.merck.comand?connect with us on?Twitter,?Facebook,?Instagram,?YouTube?and?LinkedIn.
Forward-Looking Statement of Merck & Co., Inc., Kenilworth, N.J., USA
This news release of Merck & Co., Inc., Kenilworth, N.J., USA (the “company”) includes “forward-looking statements” within the meaning of the safe harbor provisions of the U.S. Private Securities Litigation Reform Act of 1995. These statements are based upon the current beliefs and expectations of the company’s management and are subject to significant risks and uncertainties. There can be no guarantees with respect to pipeline products that the products will receive the necessary regulatory approvals or that they will prove to be commercially successful. If underlying assumptions prove inaccurate or risks or uncertainties materialize, actual results may differ materially from those set forth in the forward-looking statements.
Risks and uncertainties include but are not limited to, general industry conditions and competition; general economic factors, including interest rate and currency exchange rate fluctuations; the impact of the global outbreak of novel coronavirus disease (COVID-19); the impact of pharmaceutical industry regulation and health care legislation in the United States and internationally; global trends toward health care cost containment; technological advances, new products and patents attained by competitors; challenges inherent in new product development, including obtaining regulatory approval; the company’s ability to accurately predict future market conditions; manufacturing difficulties or delays; financial instability of international economies and sovereign risk; dependence on the effectiveness of the company’s patents and other protections for innovative products; and the exposure to litigation, including patent litigation, and/or regulatory actions.
The company undertakes no obligation to publicly update any forward-looking statement, whether as a result of new information, future events or otherwise. Additional factors that could cause results to differ materially from those described in the forward-looking statements can be found in the company’s 2019 Annual Report on Form 10-K and the company’s other filings with the Securities and Exchange Commission (SEC) available at the SEC’s Internet site (www.sec.gov).
1?GLOBOCAN2018: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 20122018.
https://gco.iarc.fr/today/data/factsheets/cancers/29-Kidney-fact-sheet.pdfhttp://globocan.iarc.fr/.
2?“Cancer Stat Facts: Kidney and Renal Pelvis Cancer.” Surveillance, Epidemiology, and End Results Program (SEER), National Cancer Institute,?https://seer.cancer.gov/statfacts/html/kidrp.html.
3?American Cancer Society. Key Statistics About Kidney Cancer,?https://www.cancer.org/cancer/kidney-cancer/about/key-statistics.html
4?“Key Statistics About Kidney Cancer.” American Cancer Society.?https://www.cancer.org/cancer/kidney-cancer/about/key-statistics.html.
5?Thomas A. Z. et al. The Role Of Metastasectomy In Patients With Renal Cell Carcinoma With Sarcomatoid Dedifferentiation: A Matched Controlled Analysis.?The Journal of Urology. 2016 Sep; 196(3): 678–684,?https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5014677/.
6?Shinder B et al. Surgical Management of Advanced and Metastatic Renal Cell Carcinoma: A Multidisciplinary Approach. Frontiers in Oncology. 2017; 7: 107.?https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5449498/#__ffn_sectitle.
7?Padala, S. A., Barsouk, A., Thandra, K. C., Saginala, K., Mohammed, A., Vakiti, A., Rawla, P., & Barsouk, A. (2020). Epidemiology of Renal Cell Carcinoma. World journal of oncology, 11(3), 79–87.?https://doi.org/10.14740/wjon1279
Biogen (Nasdaq: BIIB) and Eisai, Co., Ltd. (Tokyo, Japan) today announced that the European Medicines Agency (EMA) has confirmed it has accepted for review, following a standard timetable, the Marketing Authorization Application (MAA) for aducanumab, an investigational treatment for Alzheimer’s disease. Clinical data from patients with Mild Cognitive Impairment due to Alzheimer’s disease and mild Alzheimer’s disease demonstrate that treatment with aducanumab resulted in the removal of amyloid beta and better clinical outcomes. If approved, aducanumab would become the first therapy to reduce the clinical decline in patients with Alzheimer’s disease.
“Alzheimer’s disease has become a significant and growing burden for societies around the world, and we believe aducanumab represents the first breakthrough that can change the course of this devastating disease,” said Michel Vounatsos, Chief Executive Officer at Biogen. “We are committed to working with regulatory authorities worldwide and we look forward to the European Medicines Agency’s review of this application.”
“There are no treatments available that impact the progression of Alzheimer’s disease by addressing the underlying disease pathology. The potential that aducanumab may hold to effectively reduce the clinical decline brings new hope to people and families living with this devastating disease,” said Dr. Haruo Naito, Chief Executive Officer at Eisai Co., Ltd. “The acceptance of the Marketing Authorization Application in the European Union is an important milestone as we work towards making this potential treatment available around the world.”
Aducanumab is also under review with the U.S. Food and Drug Administration with Priority Review, with a Prescription Drug User Fee Act (PDUFA) action date of March 7, 2021.
About Aducanumab
Aducanumab (BIIB037) is an investigational human monoclonal antibody studied for the treatment of Alzheimer’s disease. Based on clinical data from patients with Mild Cognitive Impairment due to Alzheimer’s disease and mild Alzheimer’s disease, aducanumab has the potential to impact underlying disease pathophysiology, slow cognitive and functional decline and provide benefits on patients’ ability to perform activities of daily living, including conducting personal finances, performing household chores, such as cleaning, shopping and doing laundry, and independently traveling out of the home. If approved, aducanumab would be the first treatment to meaningfully change the course of the disease for individuals living with Alzheimer’s.
Biogen licensed aducanumab from Neurimmune under a collaborative development and license agreement. Since October 2017 Biogen and Eisai Co., Ltd. have collaborated on the development and commercialization of aducanumab globally.
EMERGE and ENGAGE were Phase 3 multicenter, randomized, double-blind, placebo-controlled, parallel-group studies designed to evaluate the efficacy and safety of aducanumab. The primary objective of the studies was to evaluate the efficacy of monthly doses of aducanumab as compared with placebo in reducing cognitive and functional impairment as measured by changes in the Clinical Dementia Rating-Sum of Boxes (CDR-SB) score. Secondary objectives were to assess the effect of monthly doses of aducanumab as compared to placebo on clinical decline as measured by the Mini-Mental State Examination (MMSE), Alzheimer’s Disease Assessment Scale-Cognitive Subscale 13 Items (ADAS-Cog 13) and Alzheimer’s Disease Cooperative Study-Activities of Daily Living Inventory Mild Cognitive Impairment Version (ADCS-ADL-MCI).
About Alzheimer’s Disease
Alzheimer’s disease is a progressive neurological condition that impairs thinking, memory and independence, leading to premature death. The disease currently cannot be stopped, delayed or prevented and is a growing global health crisis, affecting those living with the disease and their families. According to the World Health Organization (WHO), tens of millions of people worldwide live with Alzheimer’s disease, and the number will grow in the years ahead, outpacing the healthcare resources needed to manage it and costing billions of dollars.
The Alzheimer’s Disease International 2019 Alzheimer’s Yearbook estimates that approximately 10 million people in the EU suffer from dementia (excluding Mild Cognitive Impairment). AD is suspected to represent around 60-70% of dementia cases.
Alzheimer’s disease is characterized by changes in the brain, including the abnormal accumulation of toxic amyloid beta plaque, which begins approximately 20 years before patients exhibit symptoms of the disease. Mild Cognitive Impairment due to Alzheimer’s disease is one of the earliest stages of the disease when symptoms start to be more visible and can be detected and diagnosed. Current research efforts are focused on catching and treating patients as early as possible for the best chance of slowing or stopping the progression of Alzheimer’s disease.
??
About Biogen
At Biogen, our mission is clear: we are pioneers in neuroscience. Biogen discovers, develops and delivers worldwide innovative therapies for people living with serious neurological and neurodegenerative diseases as well as related therapeutic adjacencies. One of the world’s first global biotechnology companies, Biogen was founded in 1978 by Charles Weissmann, Heinz Schaller, Kenneth Murray and Nobel Prize winners Walter Gilbert and Phillip Sharp. Today Biogen has the leading portfolio of medicines to treat multiple sclerosis, has introduced the first approved treatment for spinal muscular atrophy, commercializes biosimilars of advanced biologics and is focused on advancing research programs in multiple sclerosis and neuroimmunology, Alzheimer’s disease and dementia, neuromuscular disorders, movement disorders, ophthalmology, immunology, neurocognitive disorders, acute neurology and pain.
We routinely post information that may be important to investors on our website at?www.biogen.com. Follow us on social media –?Twitter,?LinkedIn,?Facebook,?YouTube.
??
About Eisai Co., Ltd.
Eisai Co., Ltd. is a leading global pharmaceutical company headquartered in Japan. Eisai’s corporate philosophy is based on the?human health care (hhc)?concept, which is to give first thought to patients and their families, and to increase the benefits that health care provides to them. With a global network of R&D facilities, manufacturing sites and marketing subsidiaries, we strive to realize our?hhc?philosophy by delivering innovative products to target diseases with high unmet medical needs, with a particular focus in our strategic areas of Neurology and Oncology.
Leveraging the experience gained from the development and marketing a treatment for Alzheimer’s disease, Eisai aims to establish the “Eisai Dementia Platform.” Through this platform, Eisai plans to deliver novel benefits to those living with dementia and their families through constructing a “Dementia Ecosystem,” by collaborating with partners such as medical organizations, diagnostic development companies, research organizations, and bio-ventures in addition to private insurance agencies, finance industries, fitness clubs, automobile makers, retailers, and care facilities. For more information about Eisai Co., Ltd., please visit?https://www.eisai.com.
Biogen Safe Harbor?
This news release contains forward-looking statements, including statements made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995, about potential regulatory discussions, submissions and approvals and the timing thereof; the potential clinical effects of aducanumab; the potential benefits, safety and efficacy of aducanumab; the treatment of Alzheimer’s disease; the anticipated benefits and potential of Biogen’s collaboration arrangements with Eisai; the potential of Biogen’s commercial business and pipeline programs, including aducanumab; and risks and uncertainties associated with drug development and commercialization. These statements may be identified by words such as “aim,” “anticipate,” “believe,” “could,” “estimate,” “expect,” “forecast,” “intend,” “may,” “plan,” “possible,” “potential,” “will,” “would” and other words and terms of similar meaning. Drug development and commercialization involve a high degree of risk, and only a small number of research and development programs result in commercialization of a product. Results in early stage clinical trials may not be indicative of full results or results from later stage or larger scale clinical trials and do not ensure regulatory approval. You should not place undue reliance on these statements or the scientific data presented.
These statements involve risks and uncertainties that could cause actual results to differ materially from those reflected in such statements, including without limitation actual timing and content of submissions to and decisions made by the regulatory authorities regarding aducanumab; regulatory submissions may take longer or be more difficult to complete than expected; regulatory authorities may require additional information or further studies, or may fail or refuse to approve or may delay approval of Biogen’s drug candidates, including aducanumab; unexpected concerns that may arise from additional data, analysis or results obtained during clinical trials; the occurrence of adverse safety events; risks of unexpected costs or delays; the risk of other unexpected hurdles; uncertainty of success in the development and potential commercialization of aducanumab; risks relating to the potential launch of aducanumab, including preparedness of healthcare providers to treat patients, the ability to obtain and maintain adequate reimbursement for aducanumab and other unexpected difficulties or hurdles; failure to protect and enforce Biogen’s data, intellectual property and other proprietary rights and uncertainties relating to intellectual property claims and challenges; product liability claims; third party collaboration risks; and the direct and indirect impacts of the ongoing COVID-19 pandemic on Biogen’s business, results of operations and financial condition. The foregoing sets forth many, but not all, of the factors that could cause actual results to differ from Biogen’s expectations in any forward-looking statement. Investors should consider this cautionary statement, as well as the risk factors identified in Biogen’s most recent annual or quarterly report and in other reports Biogen has filed with the U.S. Securities and Exchange Commission. These statements are based on Biogen’s current beliefs and expectations and speak only as of the date of this news release. Biogen does not undertake any obligation to publicly update any forward-looking statements, whether as a result of new information, future developments or otherwise.
]]>Regarding lecanemab, Eisai will conduct four oral presentations. The first oral presentation will cover clinical study design and initial screening results of the newly initiated Phase III clinical study AHEAD 3-45 for preclinical Alzheimer’s disease (AD) patients. The second oral presentation will cover the latest analysis results on expression of amyloid-related imaging abnormalities-edema (ARIA-E) from the Phase II study (Study 201) conducted on early AD patients. The third oral presentation will cover changes in brain-Aβ amounts and preliminary analysis results on ARIA-E expression as observed in the first 12-month treatment period of the ongoing open-label extension (OLE) study of Study 201. The fourth oral presentation will cover baseline characteristics of currently enrolled subjects in the Phase III study Clarity AD being conducted on early AD patients.
Other presentation topics include the effectiveness of lemborexant on irregular-sleep-wake- rhythm-disorder (ISWRD) in AD as observed in mouse models in relation to clinical trials, as well results from a Phase I, First-In-Human (FIH), Single Ascending Dose (SAD) study of the novel anti-microtubule binding region (MTBR) tau antibody E2814.
Regarding aducanumab, Biogen Inc. (Headquarters: Cambridge, Massachusetts, United States) will conduct an oral presentation on the design of its Phase IIIb redosing study EMBARK. A Biologics License Application (BLA) to the U.S. Food and Drug Administration (FDA), for approval of aducanumab as an AD treatment, was accepted and received priority review designation in August 2020.
Lecanemab and aducanumab are being jointly developed by Eisai and Biogen Inc.
Regarding the joint research effort with Sysmex Corporation (Headquarters: Hyogo, “Sysmex”) for creation of simplified diagnosis of AD using blood, a poster will be presented on prediction of Amyloid Positivity Defined by Amyloid PET Centiloid through Plasma Aβ Ratio Measurement on a Fully Automated Immunoassay, HISCLTM*.
Eisai aims to realize the prevention and cure of dementia through a multi-dimensional and holistic approach with a foundation of over 35 years of experience of drug discovery activities in the area of AD and dementia. Eisai strives to create innovative medicines as soon as possible to further contribute to addressing the unmet medical needs of, as well as increasing the benefits provided to, those living with the disease and their families.
*HISCLTM?is a trademark of Sysmex Corporation.
■ Eisai oral presentation topics
| Asset in Development, Session Number |
Topic/Planned Date and Time (Eastern Standard Time) |
|---|---|
|
BAN2401 |
The AHEAD 3-45 Study of BAN2401 in Preclinical Alzheimer’s Disease: Study Design and Initial Screening Results Live oral presentation: November 4 (Wed.) 10:00 AM-10:15 AM Q&A Session: November 4 (Wed.) 11:25 AM-11:40 AM |
| BAN2401 OC 10 |
Baseline Characteristics for Clarity AD: A Phase 3 Placebo-Controlled, Double-Blind, Parallel-Group, 18-Month Study Evaluating BAN2401 in Early Alzheimer’s Disease Oral presentation available for on-demand viewing November 4 (Wed.) 1:00 AM |
| BAN2401 OC 14 |
BAN2401 and ARIA-E in Early Alzheimer’s Disease: Pharmacokinetic / Pharmacodynamic Time-to-Event Analysis From the Phase 2 Study in Early Alzheimer’s Disease Live oral presentation: November 5 (Thu.) 9:45 AM-10:00 AM Q&A Session: November 5 (Thu.) 11:15 AM-11:30 AM |
| BAN2401 LB 24 |
Preliminary Analysis of BAN2401 Effects On Brain Amyloid And ARIA-E Findings Over 12 Months Of Treatment In The Open-Label Extension Of The Phase2b Study BAN2401-G000-201 In Subjects With Early Alzheimer’s Disease Live oral presentation: November 7 (Sat.) 12:10 PM-12:25 PM Q&A Session: November 7 (Sat.) 12:25 PM-12:50 PM |
| Lemborexant LB 15 |
Irregular Sleep-Wake Rhythm Disorder in Alzheimer’s Disease: SAMP8 Mouse Strain as an Animal Model and Efficacy of the Dual Orexin (Hypocretin) Receptor Antagonist Lemborexant Oral presentation available for on-demand viewing November 6 (Fri.) 1:00 AM |
| E2814 LB 23 |
A Phase 1, First-In-Human (FIH), Single Ascending Dose (SAD) Study of the Novel Anti-Tau Therapeutic Antibody E2814?in Healthy Volunteers Live oral presentation: November 7 (Sat.) 11:55 AM-12:10 PM Q&A Session: November 7 (Sat.) 12:25 PM-12:50 PM |
■ Eisai poster presentation topics
| Asset in Development, Poster Number |
Topic/Planned Date and Time (Eastern Standard Time) |
|---|---|
| General P 60 |
Congruence of Clinical Assessment Instruments with Online Narratives Over Social Media by Patients with Alzheimer’s Disease and Their Caregivers Available for on-demand viewing beginning November 4 (Wed.) |
■ Biogen oral presentation topics
| Asset in Development, Session Number? |
Topic/Planned Date and Time (Eastern Standard Time)? |
|---|---|
| Aducanumab OC 3 |
EMBARK: A Phase 3b, Open-Label, Single-Arm, Safety Study to Evaluate the Long-Term Safety and Efficacy of Aducanumab in Eligible Participants with Alzheimer’s Disease Live oral presentation: November 4 (Wed.) 10:15 AM-10:30 AM Q&A Session: November 4 (Wed.) 11:25 AM-11:40 AM |
■ Biogen poster presentation topics
| Asset in Development, Poster Number? |
Topic/Planned Date and Time (Eastern Standard Time)? |
|---|---|
| General P 70 |
Estimating progression rates across the spectrum of Alzheimer’s disease for amyloid positive individuals using National Alzheimer’s Coordinating Center data Available for on-demand viewing beginning November 4 (Wed.) |
■ Sysmex-Eisai poster presentation topics
| Asset in Development, Poster Number? |
Topic/Planned Date and Time (Eastern Standard Time)? |
|---|---|
| General LP 10 |
Plasma Aβ Ratio Measured on a Fully Automated Immunoassay Predicts Amyloid Positivity Defined by Amyloid PET Centiloid Available for on-demand viewing beginning November 4 (Wed.) |
Media Inquiries:
Public Relations Department,
Eisai Co., Ltd.
+81-(0)3-3817-5120
[Notes to editors]
1. About the Joint Development Agreement between Eisai and Biogen for AD
Eisai and Biogen are widely collaborating on the joint development and commercialization of AD treatments. Eisai serves as the lead in the co-development of lecanemab (Development Code: BAN2401), an anti-Aβ protofibril antibody, while Biogen serves as the lead for co-development of aducanumab, Biogen’s investigational anti-Aβ antibody for patients with AD, and the companies plan to pursue marketing authorizations for the two compounds worldwide. If approved, the companies will also co-promote the products in major markets, such as the United States, the European Union and Japan.
?
2. About the collaboration between Eisai and Sysmex
Eisai and Sysmex have entered into a comprehensive non-exclusive collaboration agreement aimed at the creation of new diagnostics in the field of dementia in February 2016. Leveraging each other’s technologies and knowledge, the two companies aim to discover next-generation diagnostics that will enable early diagnosis, selection of treatment options and the regular monitoring of the effects of treatment for dementia.
3. About lecanemab (Development Code: BAN2401)
Lecanemab is a humanized monoclonal antibody for AD that is the result of a strategic research alliance between Eisai and BioArctic AB (Headquarters: Sweden). Lecanemab selectively binds to neutralize and eliminate soluble, toxic Aβ aggregates (protofibril) that are thought to contribute to the neurodegenerative process in AD. As such, lecanemab may have the potential to have an effect on disease pathology and to slow down the progression of the disease. Eisai obtained the global rights to study, develop, manufacture and market lecanemab for the treatment of AD pursuant to an agreement concluded with BioArctic in December 2007. Currently, a global clinical Phase III study (Clarity AD) of lecanemab in early AD is underway. Lecanemab is being jointly developed by Eisai and Biogen Inc. The National Institutes of Health, National Institute of Aging are providing funding for the A45 Study (grant number R01AG061848) and A3 Study (grant number R01AG054029).
4. About Lemborexant
Lemborexant, an orexin receptor antagonist, is Eisai’s in-house discovered and developed small molecule that inhibits orexin neurotransmission by binding competitively to the two subtypes of orexin receptors (orexin receptor 1 and 2). Faster on/off receptor kinetics of lemborexant to orexin receptor 2, which also suppresses non-REM sleep, may influence lemborexant’s potential to facilitate improvements in sleep onset and maintenance. In June 2020, lemborexant was launched under the product name DAYVIGOTM?in the U.S. for the treatment of adult patients with insomnia, characterized by difficulties with sleep onset and/or sleep maintenance; and in July 2020, it was launched under the product name DAYVIGO? in Japan for the treatment of insomnia. Eisai has submitted new drug applications seeking approval of DAYVIGO in Canada, Australia and HKSA. In addition, a Phase II clinical study of lemborexant in patients with ISWRD associated with mild to moderate Alzheimer’s dementia is underway.
5. About Aducanumab (Development Code: BIIB037)
Aducanumab is an investigational human monoclonal antibody studied for the treatment of AD. Based on clinical data, aducanumab has the potential to impact underlying disease pathophysiology, slow cognitive and functional decline and provide benefits on patients’ ability to perform activities of daily living, including conducting personal finances, performing household chores, such as cleaning, shopping and doing laundry, and independently traveling out of the home. If approved, aducanumab would be the first treatment to meaningfully change the course of the disease for individuals living with Alzheimer’s.
Biogen licensed aducanumab from Neurimmune under a collaborative development and license agreement. Since October 2017 Biogen and Eisai have collaborated on the development and commercialization of aducanumab globally.
EMERGE and ENGAGE were Phase III multicenter, randomized, double-blind, placebo-controlled, parallel-group studies designed to evaluate the efficacy and safety of aducanumab. The primary objective of the studies was to evaluate the efficacy of monthly doses of aducanumab as compared with placebo in reducing cognitive and functional impairment as measured by changes in the Clinical Dementia Rating-Sum of Boxes (CDR-SB) score. Secondary objectives were to assess the effect of monthly doses of aducanumab as compared to placebo on clinical decline as measured by the Mini-Mental State Examination (MMSE), Alzheimer’s Disease Assessment Scale-Cognitive Subscale 13 Items (ADAS-Cog 13) and Alzheimer’s Disease Cooperative Study-Activities of Daily Living Inventory Mild Cognitive Impairment Version (ADCS-ADL-MCI).
]]>AMGEN’S APREMILAST AND EISAI’S ERITORAN TO BE EVALUATED ACROSS MULTIPLE INTERNATIONAL TRIAL SITES WITHIN THE REMAP NETWORK
(BUSINESS WIRE)–Global Coalition for Adaptive Research (LOS ANGELES, CA), Amgen (THOUSAND OAKS, CA),?and Eisai Co., Ltd. (TOKYO, Japan “Eisai”) — The Global Coalition for Adaptive Research (GCAR) in collaboration with Amgen and Eisai, today announced enrollment of the first patient in the immune modulation domain of REMAP-COVID, a sub-study of REMAP-CAP (A?Randomized,?Embedded,?Multifactorial,?Adaptive?Platform trial for?Community-Acquired?Pneumonia) that tests multiple interventions for the treatment of patients hospitalized with COVID-19. Amgen’s?apremilast?and Eisai’s investigational?eritoran?are being evaluated as potential therapeutic agents.
REMAP-CAP was developed to test treatments for severe pneumonia both in non-pandemic and pandemic settings. In February 2020, REMAP-CAP rapidly pivoted to its pandemic mode (the REMAP-COVID sub-study), as per its original intent, to incorporate additional potential treatment regimens specifically targeting COVID-19 and to expand enrollment to COVID-19 patients. This trial is a multicenter, randomized platform study, with treatments tested within groupings or “domains” based on pathway or mechanism of action.
The trial is being conducted in the multi-hospital UPMC (University of Pittsburgh Medical Center) health system along with over 20 hospitals in the United States. Additional global sites across the trial network will follow. University of Pittsburgh is serving as the U.S. Regional Coordinating Center.
“Partnering with the biopharmaceutical industry to be able to efficiently test well-understood targeted agents is critical to understanding treatment paradigms for COVID-19 patients,” says Derek Angus, MD., MPH, FRCP, U.S. Principal Investigator of REMAP and Chief Healthcare Innovation Officer, UPMC Health System. “Today’s announcement marks an important milestone in the collaboration between industry and the scientific and academic community to work collectively to evaluate potentially promising therapies to support patients hospitalized with COVID-19.”
Amgen’s?apremilast?is an oral drug which inhibits the activity of PDE4 (Phosphodiesterase 4), an enzyme found in inflammatory cells in the human body. By inhibiting PDE4,?apremilast?is thought to modulate the production of inflammatory cytokines and other mediators, which may prove helpful in inhibiting the inflammatory response associated with the signs, symptoms and pulmonary involvements observed in some COVID-19 patients.?Apremilast?is currently approved for use in more than 45 countries as an oral treatment for inflammatory diseases including moderate to severe plaque psoriasis, psoriatic arthritis and oral ulcers associated with Behcet’s disease.
“Amgen believes that, based on its mechanism of action,?apremilast?might help prevent the respiratory distress seen in moderate to severe-stage adult COVID-19 patients,” said David M. Reese, M.D., Executive Vice President of Research and Development at Amgen. “We are proud to be joining REMAP-COVID, which is an important and innovative effort utilizing a platform approach and has the?potential to rapidly identify whether?apremilast?may improve health outcomes for patients hospitalized with moderate to severe COVID-19.”
Eritoran?is Eisai’s in-house discovered and developed investigational TLR4 (Toll-Like Receptor 4) antagonist created with natural product organic synthesis technology. It is a structural analogue of Lipid A, which is an activator of endotoxins of bacteria. It has been previously observed to be safe in 14 clinical studies including a large Phase 3 randomized trial in severe sepsis. It is expected to suppress inflammation and increasing in severity caused by COVID-19 by inhibiting the activation of TLR4, which is found in the most upstream of various cytokine gene expression signaling that causes the cytokine-storm.
“Eisai is pleased to participate in the groundbreaking REMAP-COVID effort, and we expect that this study will generate important insights about?eritoran’s?potential to possibly improve health outcomes for patients with moderate and severe COVID-19,” said Lynn Kramer, M.D., FAAN, Chief Clinical Officer, Neurology Business Group, Eisai “As part of our human health care mission, we are committed to making a difference for patients, their families and health care professionals across the globe.”
GCAR is the U.S. Sponsor of REMAP-COVID and is guiding efforts to facilitate the inclusion of multiple pharma partners in REMAP-COVID globally.
“GCAR is delighted to utilize our expertise in implementing and overseeing innovative trials to collaborate on this important effort,” shared Meredith Buxton, PhD, Chief Executive Officer of GCAR.? “We are committed to working closely with pharma and the REMAP Network to identify new effective treatments for patients with COVID-19 by serving as U.S. sponsor of this important and innovative platform trial.
About REMAP-CAP
REMAP-CAP is led by world experts in critical care, clinical trials, pandemic and infectious disease outbreaks, virology, immunology, emergency medicine, and Bayesian statistics. REMAP-CAP has enrolled over 2000 patients at 263 sites across 19 countries. This vital research is being conducted in collaboration with Berry Consultants, leaders in statistical design for adaptive platform trials, and is being supported by governments and non-profits worldwide.
To learn more about REMAP-CAP and the REMAP-COVID sub-study, please visit?www.remapcap.org?and follow?@remap_cap
About Otezla??(apremilast)
Otezla??(apremilast) is an oral small-molecule inhibitor of phosphodiesterase 4 (PDE4) specific for cyclic adenosine monophosphate (cAMP). PDE4 inhibition results in increased intracellular cAMP levels, which is thought to indirectly modulate the production of inflammatory mediators. The specific mechanism(s) by which Otezla exerts its therapeutic action in patients is not well defined.
By inhibiting PDE4, Otezla is thought to modulate the production of inflammatory cytokines and other mediators, which may prove helpful in inhibiting the inflammatory response associated with the signs, symptoms and pulmonary involvements observed in some COVID-19 patients. Amgen plans to collaborate with platform trials to investigate Otezla in treatment of hospitalized COVID-19 patients.
Otezla??(apremilast)?U.S.?INDICATIONS
Otezla??(apremilast) is indicated for the treatment of adult patients with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy.
Otezla is indicated for the treatment of adult patients with active psoriatic arthritis.
Otezla is indicated for the treatment of adult patients with oral ulcers associated with Beh?et’s?Disease.
Otezla??(apremilast)?U.S.?IMPORTANT SAFETY INFORMATION
Contraindications
- Otezla?(apremilast) is contraindicated in patients with a known hypersensitivity to apremilast or to any of the excipients in the formulation
Warnings and Precautions
- Diarrhea, Nausea, and Vomiting: Cases of severe diarrhea, nausea, and vomiting were associated with the use of Otezla. Most events occurred within the first few weeks of treatment. In some cases patients were hospitalized. Patients 65 years of age or older and patients taking medications that can lead to volume depletion or hypotension may be at a higher risk of complications from severe diarrhea, nausea, or vomiting. Monitor patients who are more susceptible to complications of diarrhea or vomiting; advise patients to contact their healthcare provider. Consider Otezla dose reduction or suspension if patients develop severe diarrhea, nausea, or vomiting
- Depression: Carefully weigh the risks and benefits of treatment with Otezla for patients with a history of depression and/or suicidal thoughts/behavior, or in patients who develop such symptoms while on Otezla. Patients, caregivers, and families should be advised of the need to be alert for the emergence or worsening of depression, suicidal thoughts or other mood changes, and they should contact their healthcare provider if such changes occur
? o?Psoriasis:?Treatment with Otezla is associated with an increase in depression. During clinical trials, 1.3% (12/920) of patients reported depression compared to 0.4% (2/506) on placebo. Depression was reported as serious in 0.1% (1/1308) of patients exposed to Otezla, compared to none in placebo-treated patients (0/506). Suicidal behavior was observed in 0.1% (1/1308) of patients on Otezla, compared to 0.2% (1/506) on placebo. One patient treated with Otezla attempted suicide; one patient on placebo committed suicide
? o?Psoriatic Arthritis:?Treatment with Otezla is associated with an increase in depression. During clinical trials, 1.0% (10/998) reported depression or depressed mood compared to 0.8% (4/495) treated with placebo. Suicidal ideation and behavior was observed in 0.2% (3/1441) of patients on Otezla, compared to none in placebo-treated patients. Depression was reported as serious in 0.2% (3/1441) of patients exposed to Otezla, compared to none in placebo-treated patients (0/495). Two patients who received placebo committed suicide compared to none on Otezla
? o??Behcet’s Disease:?Treatment with Otezla is associated with an increase in depression. During the phase 3 clinical trial, 1% (1/104) reported depression or depressed mood compared to 1% (1/103) treated with placebo. No instances of suicidal ideation or behavior were reported in patients treated with Otezla or treated with placebo
- Weight Decrease: Monitor body weight regularly; evaluate unexplained or clinically significant weight loss, and consider discontinuation of Otezla
? o??Psoriasis:?During the clinical trials, body weight loss of 5-10% occurred in 12% (96/784) of patients treated with Otezla and in 5% (19/382) of patients treated with placebo. Body weight loss of ≥10% occurred in 2% (16/784) of patients treated with Otezla compared to 1% (3/382) of patients treated with placebo
? o??Psoriatic Arthritis:?During the clinical trials, body weight loss of 5-10% was reported in 10% (49/497) of patients taking Otezla and in 3.3% (16/495) of patients taking placebo
? o??Behcet’s Disease:?During the clinical trials, body weight loss of >5% was reported in 4.9% (5/103) of patients taking Otezla and in 3.9% (4/102) of patients taking placebo
- Drug Interactions: Apremilast exposure was decreased when Otezla was co-administered with rifampin, a strong?CYP450?enzyme inducer; loss of Otezla efficacy may occur. Concomitant use of Otezla with?CYP450?enzyme inducers (e.g., rifampin, phenobarbital, carbamazepine, phenytoin) is not recommended
Adverse Reactions
- Psoriasis:?Adverse reactions reported in ≥5% of patients were (Otezla%, placebo%): diarrhea (17, 6), nausea (17, 7), upper respiratory tract infection (9, 6), tension headache (8, 4), and headache (6, 4)
- Psoriatic Arthritis:?Adverse reactions reported in at least 2% of patients taking Otezla, that occurred at a frequency at least 1% higher than that observed in patients taking placebo, for up to 16 weeks (after the initial 5-day titration), were (Otezla%, placebo%): diarrhea (7.7, 1.6); nausea (8.9, 3.1); headache (5.9, 2.2); upper respiratory tract infection (3.9, 1.8); vomiting (3.2, 0.4); nasopharyngitis (2.6, 1.6); upper abdominal pain (2.0, 0.2)
- Beh?et’s Disease:?Adverse reactions reported in at least ≥5% of patients taking Otezla, that occurred at a frequency at least 1% higher than that observed in patients taking placebo, for up to 12 weeks, were (Otezla%, placebo%): diarrhea (41.3, 20.4); nausea (19.2, 10.7); headache (14.4, 10.7); upper respiratory tract infection (11.5, 4.9); upper abdominal pain (8.7, 1.9); vomiting (8.7, 1.9); back pain (7.7, 5.8); viral upper respiratory tract infection (6.7, 4.9); arthralgia (5.8, 2.9)
Use in Specific Populations
- Pregnancy: Otezla has not been studied in pregnant women. Advise pregnant women of the potential risk of fetal loss. Consider pregnancy planning and prevention for females of reproductive potential. There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to Otezla during pregnancy. Information about the registry can be obtained by calling 1-877-311-8972 or visiting?https://mothertobaby.org/ongoing-study/otezla/
- Lactation: There are no data on the presence of apremilast or its metabolites in human milk, the effects of apremilast on the breastfed infant, or the effects of the drug on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Otezla and any potential adverse effects on the breastfed child from Otezla or from the underlying maternal condition
- Renal Impairment: Otezla dosage should be reduced in patients with severe renal impairment (creatinine clearance less than 30 mL/min) for details, see Dosage and Administration, Section 2, in the Full Prescribing Information
Please click?here?for Otezla??Full Prescribing Information.
About Eritoran (E5564)
Eritoran is Eisai’s in-house discovered and developed investigational TLR4 (Toll-Like Receptor 4) antagonist created with natural product organic synthesis technology. It is a structural analogue of Lipid A which is an activator of endotoxins of bacteria. It has been previously observed to be safe in 14 clinical studies including a large Phase 3 randomized trial in severe sepsis. It is expected to suppress inflammation and increasing in severity caused by COVID-19 by inhibiting the activation of TLR4, which is found in the most upstream of various cytokine gene expression signaling that causes the cytokine-storm.
About Global Coalition for Adaptive Research (GCAR)
The Global Coalition for Adaptive Research (GCAR) is a 501(c)(3) nonprofit organization uniting physicians, clinical researchers, advocacy and philanthropic organizations, biopharma, health authorities, and other key stakeholders in healthcare to expedite the discovery and development of treatments for patients with rare and deadly diseases by serving as Sponsor of innovative and complex trials including master protocols and platform trials. In this effort, GCAR is serving as U.S. Trial Sponsor of REMAP-CAP.
To learn more about GCAR, visit?www.gcaresearch.org?and follow us: @GCAResearch and?www.facebook.com/GCAResearch.
About UPMC (University of Pittsburgh Medical Center)
Pittsburgh-based UPMC is inventing new models of patient-centered, cost-effective, accountable care. UPMC integrates more than 90,000 employees, 40 hospitals, 700 doctors’ offices and outpatient sites.?U.S. News & World Report?consistently ranks UPMC Presbyterian Shadyside on its annual Honor Roll of America’s Best Hospitals and ranks UPMC Children’s Hospital of Pittsburgh on its Honor Roll of America’s Best Children’s Hospitals.?For more information, go to?UPMC.com.
About?Amgen?
Amgen?is committed to unlocking the potential of biology for patients suffering from serious illnesses by discovering, developing, manufacturing and delivering innovative human therapeutics. This approach begins by using tools like advanced human genetics to unravel the complexities of disease and understand the fundamentals of human biology.
Amgen?focuses on areas of high unmet medical need and leverages its biologics manufacturing expertise to strive for solutions that improve health outcomes and dramatically improve people’s lives. A biotechnology pioneer since 1980,?Amgen?has grown to be the world’s largest independent biotec
]]>Eisai Co., Ltd. (Headquarters: Tokyo, CEO: Haruo Naito, “Eisai”) and Cogstate, Ltd. (Headquarters: Melbourne, Australia and New Haven, USA, CEO: Brad O’Connor, “Cogstate”) announced today that the companies have entered into a collaboration whereby Eisai has secured the global development rights and exclusive commercialization rights of all cognitive function tests developed by Cogstate, including the “Cogstate Brief BatteryTM” (CBB) for use in healthcare and other markets. This global licensing deal is an expansion of an existing partnership executed in August 2019 whereby Eisai secured exclusive development and commercialization rights in Japan for all cognitive function tests developed by Cogstate, including the CBB. Both companies plan to proceed with development globally of CBB as a tool for individuals to self-assess brain performance to support healthy lifestyle choices and preventative measures in daily life, as well as a medical device to aid healthcare professionals in clinical diagnosis decisions.
Developed by Cogstate, the CBB is a scientifically validated digital tool that enables cognitive function self-checks and consists of four tests evaluating psychomotor function, attention, learning and memory, and working memory. In the United States, Europe, Australia, New Zealand and Canada, the CBB has been adapted as a medical device named “CognigramTM” that has achieved marketing authorization by regulators in these jurisdictions and provides informative results for healthcare professionals to support clinical examination to aid in the diagnosis of MCI and dementia.
In its medium-term business plan, EWAY2025, Eisai is aiming to become a “Medico Societal Innovator” (a company that changes society through creating medicines and providing solutions). Eisai is creating next-generation medical remedies focused on the neurology and oncology areas as well as building disease ecosystem platforms, in order to provide environments and solutions including digital solutions for early diagnosis and early treatment.
Cogstate aims to make assessment of brain health as simple, common and informative as assessment of blood pressure. Cogstate’s technology, which is easy to use and available in over 70 languages, is supported by extensive scientific validation, including more than 600 peer reviewed publications. Cogstate technology has been used extensively in clinical trials, including trials conducted by Eisai.
The global agreement between Eisai and Cogstate will allow the two companies to replicate many of the advancements that have already been launched in Japan, where Eisai has developed and launched a new digital tool using the CBB, named “NouKNOWTM” (pronounced “NOH-NOH”), a non-medical device for self-assessment of brain performance (brain health). Eisai is currently investigating the possibility of developing a medical device using the CBB in Japan.
In recent years, various research has demonstrated the possibility that decline in brain performance may be mitigated through major readjustments to lifestyle, such as regular exercise and sleep, a well-balanced diet, and social interaction. However, according to a survey by Eisai, the number of people taking correct preventive actions or habitually performing self-checks of cognitive function are few, with disparities (“chasms”) existing against the incorporation of these habits into daily lifestyle.
As a result of these findings, in Japan, Eisai is constructing a dementia platform, called “Easiit”, with the goal of erasing these chasms. Core to this platform will be the brain-performance self-check tool “NouKNOW” and the “Easiit App” which aims to contribute to the promotion of health practices through data visualization of brain and body health insights.
It can be expected that the use of “NouKNOW” to perform periodic self-assessments of brain performance among the generation currently in their prime working years, along with the use of the “Easiit App” for the adjustment of lifestyle and practice of preventive measures in daily life, will become an opportunity for the creation of better brain and body health.
Through this agreement, Eisai and Cogstate will work together for the development of digital tools for simpler self-checks of cognitive function and diagnostic tool development for healthcare professionals to promote greater awareness of brain performance globally, thus contributing to the realization of well-being for all.
[Notes to editors]
1. About Eisai Co., Ltd.
Eisai Co., Ltd. defines our corporate mission as “giving first thought to patients and their families and to increasing the benefits health care provides,” which we call our?human health care?(hhc) philosophy. With approximately 10,000 employees working across our global network of R&D facilities, manufacturing sites and marketing subsidiaries, we strive to realize our?hhc?philosophy by delivering innovative products to address unmet medical needs, with a particular focus in our strategic areas of Neurology and Oncology. As a global pharmaceutical company, our mission extends to patients around the world through working with key stakeholders to improve access to medicines in developing and emerging countries.
For further information on Eisai Co., Ltd., please visit?https://www.eisai.com
2. About Cogstate Ltd.
Cogstate Ltd. (ASX:CGS) is a neuroscience technology company headquartered in Melbourne, Victoria, Australia and New Haven, Connecticut, USA, focused on optimising brain health assessments to advance the development of new medicines and to enable earlier clinical insights in healthcare. Since 1999, Cogstate technologies have provided rapid, reliable and highly sensitive computerised cognitive tests across a growing list of domains. The company’s clinical trials solutions include quality assurance services for clinical outcome assessments that combine electronic data capture, innovative operational approaches, advanced analytics and scientific consulting. For more than 20 years, Cogstate has proudly supported the leading-edge research needs of biopharmaceutical companies and academic institutions and the clinical care needs of physicians and patients around the world. Notwithstanding this agreement, Cogstate will continue to independently offer its technology and services to the clinical trials market.
For further information on Cogstate, please visit?www.cogstate.com.
Media Inquiries:
Public Relations Department,
Eisai Co., Ltd.
+81-(0)3-3817-5120
The submission covering monotherapy for partial-onset seizures was based on subgroup analysis estimating monotherapy safety and efficacy within clinical studies of the combination therapy (Study 304, 305, 306, and 335) conducted globally including the United States, Europe and China on patients ages 12 years and older with partial-onset seizures (with or without secondarily generalized seizures). Additionally, results of a Phase III clinical study (FREEDOM/Study 342) conducted in Japan and South Korea on untreated epilepsy patients ages 12 years to 74 years old with partial-onset seizures (with or without secondarily generalized seizures) were submitted as supplementary safety and efficacy data.
The submission covering partial-onset seizures in pediatric patients was based on the results of a Phase III clinical study (Study 311) of Fycompa as adjunctive therapy conducted globally on pediatric patients (ages 4 to less than 12 years) with inadequately controlled partial-onset seizures or primary generalized tonic-clonic seizures.
In China, it is estimated that there are approximately 9 million patients with epilepsy, and although onset occurs at any age, onset is most common in people aged 18 and younger and the elderly. As approximately 30% of patients with epilepsy are unable to control their seizures with currently available AEDs1, this is a disease with significant unmet medical needs.
Fycompa is a first-in-class AED and a once-daily tablet discovered at Eisai’s Tsukuba Research Laboratories. The agent is a highly selective, noncompetitive AMPA receptor antagonist that reduces neuronal hyper-excitation associated with seizures by targeting glutamate activity at AMPA receptors on postsynaptic membranes. Fycompa has been approved in China as an adjunctive treatment for partial-onset seizures with or without secondarily generalized seizures in patients with epilepsy 12 years of age and older.
Eisai considers neurology, including epilepsy, a therapeutic area of focus. With the acceptance of these additional applications regarding Fycompa in China, Eisai pursues its mission to provide “seizure freedom” to a greater number of patients with epilepsy across the world. Eisai seeks to address the diverse needs of, as well as increasing the benefits provided to, patients with epilepsy and their families.
Media Inquiries:
Public Relations Department,
Eisai Co., Ltd.
+81-(0)3-3817-5120
[Notes to editors]
1. About Fycompa (generic name: perampanel)
Fycompa is a first-in-class AED discovered and developed by Eisai. With epileptic seizures being mediated by the neurotransmitter glutamate, the agent is a highly selective, noncompetitive AMPA receptor antagonist that reduces neuronal hyperexcitation associated with seizures by targeting glutamate activity at AMPA receptors on postsynaptic membranes. Fycompa is available in drug form to be taken once daily orally at bedtime. A tablet and fine granule formulation have been approved in Japan. An oral suspension formulation and tablet have been approved in the United States and Europe.
Fycompa is currently approved in more than 70 countries and territories, including Japan, the United States, China, and other countries in Europe and in Asia as an adjunctive treatment for partial-onset seizures (with or without secondarily generalized seizures) in patients with epilepsy 12 years of age and older. In addition, Fycompa has been approved in more than 65 countries, including the United States, Japan, in Europe and in Asia for treatment as an adjunctive therapy for primary generalized tonic-clonic seizures in patients with epilepsy 12 years of age and older. In Japan, the United States, and South Korea, Fycompa is approved for monotherapy and adjunctive use in the treatment of partial-onset seizures (with or without secondarily generalized seizures) in patients with epilepsy 4 years of age and older. In Europe, an application has been submitted seeking the additional approval of Fycompa for adjunctive use in the treatment of partial-onset seizures (with or without secondarily generalized seizures) or primarily generalized tonic-clonic seizures in pediatric patients with epilepsy. To date, Fycompa has been used to treat more than 300,000 patients worldwide across all indications.
Eisai is conducting a global Phase III clinical study (Study 338) for the agent in patients with seizures associated with Lennox-Gastaut syndrome. In addition, Eisai is conducting development of an injection formulation.
2. About the Phase III clinical studies upon which the additional submission in China covering monotherapy for partial-onset seizures was based
The additional submission covering monotherapy for Fycompa in China was based on the results of a Phase III clinical study (Study 3352) conducted including Japan, China, and South Korea, as well as the results of three Phase III clinical studies (Study 3043, 3054, and 3065)?conducted globally including the United States, Europe and China.
Study 335 was conducted to evaluate the efficacy and safety of Fycompa mainly for the patients in Asia region. Furthermore, Studies 304 and 305 included three arms (placebo, Fycompa 8 mg, and 12 mg) and were to evaluate a more extended dose range. The key goal of Study 306 was to identify the minimal effective dose and included four treatment arms (placebo, Fycompa 2 mg, 4 mg, and 8 mg).
These studies were conducted as the multicenter, randomized, double-blind, placebo-controlled, parallel-group study for the patients aged 12 years and older who have a diagnosis of epilepsy with partial-onset seizures receiving one to a maximum of three anti-epileptic drugs. The primary endpoint of Study 335 was the percentage change in seizure frequency. The primary endpoint of Study 304, 305, and 306 for the approval in Europe was the 50% responder rate (percentage of patients achieving a 50% or greater reduction in seizure frequency compared to pre-randomization phase), while for the approval in the United States it was the percentage change in seizure frequency. Specifically, the results showed:
1) Study 335
- The percentage changes in seizure frequency shown were -17.3% (p=0.223), -29.0% (p=0.0003), -38.0% (p<0.00001) in the 4, 8, and 12 mg Fycompa / day groups, respectively, versus -10.8% with placebo.
- The most common three adverse events were dizziness, somnolence, and nasopharyngitis.
2) Study 304
- The 50% responder rates compared to placebo were 37.6% (p=0.0760) and 36.1% (p=0.0914) in the 8 mg and 12 mg Fycompa / day groups, respectively, versus 26.4% with placebo.
- The percentage changes in seizure frequency shown were -26.3% (p=0.0261) and -34.5% (p=0.0158) in the 8 mg and 12 mg Fycompa / day groups, respectively, versus -21.0% with placebo.
- The most common six adverse events were dizziness, somnolence, irritability, headache, falls and ataxia.
3) Study 305
- The 50% responder rates compared to placebo were 33.3% (p=0.0018) and 33.9% (p=0.0006) in the 8 mg and 12 mg Fycompa / day groups, respectively, versus 14.7% with placebo.
- The percentage changes in seizure frequency shown were -30.5% (p=0.0008) and -17.6% (p=0.0105) in the 8 mg and 12 mg Fycompa / day groups, respectively, versus -9.7% with placebo.
- The most common four adverse events were dizziness, fatigue, headache, and somnolence.
4) Study 306
- The 50% responder rates compared to placebo were 20.6% (p=0.4863), 28.5% (p=0.0132), and 34.9% (p=0.0003) in the 2, 4, and 8 mg Fycompa / day groups, respectively, versus 17.9% with placebo.
- The percentage changes in seizure frequency shown were -13.6% (p=0.4197), -23.3% (p=0.0026), and -30.8% (p<0.0001), in the 2, 4, and 8 mg Fycompa / day groups, respectively, versus -10.7% with placebo.
- The most common three adverse events were dizziness, headache, and somnolence.
3. About FREEDOM (Study 342)6
FREEDOM (Study 342) is an uncontrolled, open-label Phase III clinical study evaluating efficacy and safety for the Fycompa monotherapy conducted in Japan and South Korea on untreated epilepsy patients aged 12 to 74 with partial-onset seizures with or without secondarily generalized seizures. Up to 4 mg of Fycompa was taken orally once daily before bedtime (may be titrated up to 8 mg if seizures occur). This study comprised a treatment phase including a titration period of 6 weeks and a maintenance period of 26 weeks (if titrated up from 4 mg to 8 mg, titration period was 4 weeks and maintenance period was 26 weeks) and an extension phase. In this study, 89 patients were administered Fycompa as monotherapy, and the proportion of 73 patients for evaluation receiving 4 mg who were seizure-free during the treatment period exceeded the efficacy criteria*, and the primary endpoint was met. In addition, the interim results demonstrated that the 4 mg and 8 mg patients combined also exceeded the efficacy criteria. The most common adverse events (incidence of 10% or higher) observed in this study were dizziness, somnolence, nasopharyngitis and headache, which is consistent with the safety profile of Fycompa to date.
* The criteria for efficacy in this study with 73 patients for evaluation of efficacy required a 52.1% or higher proportion of patients to have achieved seizure freedom, which was set primarily in consideration of the results from other AED monotherapy studies.
4. About Study 3117
Study 311 is a global (United States, Europe, Japan, South Korea), open-label Phase III clinical study evaluating the safety, tolerability, and exposure efficacy relationship of the Fycompa oral suspension when administered as an adjunctive therapy in 180 pediatric epilepsy patients aged 4 to less than 12 with inadequately controlled partial-onset seizures or primary generalized tonic-clonic seizures. This study comprised a treatment phase including a titration period of up to 11 weeks and a maintenance period of up to 12 weeks and an extension phase. In this study, 2 to 16 mg of Fycompa was taken orally once daily before bedtime. Primary endpoints were safety and tolerability. Efficacy was similar to that observed in patients 12 years of age and older. The most common adverse events (incidence of 10% or higher) observed in this study were somnolence, nasopharyngitis, pyrexia, vomiting, dizziness, influenza, and irritability, which is consistent with the safety profile of Fycompa to date.
5. About Epilepsy
Epilepsy affects approximately 9 million people in China, 1 million people in Japan, 3.4 million people in the United States, 6 million people in Europe, and approximately 60 million people worldwide. As approximately 30% of patients with epilepsy are unable to control their seizures with currently available AEDs,1?this is a disease with significant unmet medical needs.
Epilepsy is broadly categorized by seizure type, with partial-onset seizures accounting for approximately 60% of epilepsy cases and generalized seizures accounting for approximately 40%. In a partial-onset seizure, an abnormal electrical disturbance occurs in a limited area of the brain, and may subsequently spread throughout the brain, becoming a generalized seizure (known as a secondarily generalized seizure). In a generalized seizure, abnormal electrical disturbances occur throughout the brain, and can be followed by a loss of consciousness or physical symptoms manifested throughout the whole body.
1?“The Epilepsies and Seizures: Hope Through Research. What are the epilepsies?” National Institute of Neurological Disorders and Stroke, accessed May 24, 2016,?http://www.ninds.nih.gov/disorders/epilepsy/detail_epilepsy.htm#230253109
2?Nishida T, et al. Adjunctive perampanel in partial-onset seizures: Asia-Pacific, randomized phase III study.?Acta Neurol Scand.?2018;137:392–399.
3?French JA, et al. Adjunctive perampanel for refractory partial-onset seizures: randomized phase III study 304.?Neurology?2012; 79, 589-596
4?French JA, et al. Evaluation of adjunctive perampanel in patients with refractory partial-onset seizures: results of randomized global phase III study 305.?Epilepsia?2013; 54, 117-125.
5?Krauss GL, et al. Randomized phase III study 306: adjunctive perampanel for refractory partial-onset seizures.?Neurology?2012; 78, 1408-1415.
6?Yamamoto, Takamichi et al. Efficacy and safety of perampanel monotherapy in patients with focal-onset seizures with newly diagnosed epilepsy or recurrence of epilepsy after a period of remission: The open-label Study 342 (FREEDOM Study).?Epilepsia, 2020; 5, 274-284.
7?Fogarasi, Andras et al. Open-label study to investigate the safety and efficacy of adjunctive perampanel in pediatric patients (4 to <12 years) with inadequately controlled focal seizures or generalized tonic-clonic seizures.?Epilepsia. 2020; 61, 125-137.
The University of Tokyo (President: Makoto Gonokami, “The University of Tokyo”) and Eisai Co., Ltd. (Headquarters: Tokyo, CEO: Haruo Naito, “Eisai”) announced today a collaboration aiming for the development and drug discovery of targeted protein degradation technology has been created, with the establishment of a social cooperation program, “Protein Degradation Drug Discovery”. The research time span will last five years from October 1, 2020 to September 30, 2025.
The social cooperation program is established and operated based on funds of private organizations dedicated to conducting research in collaboration with the University of Tokyo regarding shared issues of high common concern.
The “Protein Degradation Drug Discovery” course is to be established within the Graduate School of Pharmaceutical Sciences, the University of Tokyo. Dr. Mikihiko Naito, Former Director of the Division of Molecular Target and Gene Therapy Products, National Institute of Health Sciences, has been inaugurated as a project professor for this program and will lead research with protein degradation technology including SNIPER. This research will combine the world’s most advanced ubiquitin-proteasome research as conducted in the graduate school with drug discovery knowledge fostered by Eisai, for the development of new protein degradation technology towards proteins targeted by drugs and the promotion of drug discovery research based on this technology. In addition, through this research, the course will educate and train the next generation of leaders in this research field.
Targeted protein degradation is a series of technologies in which precisely designed compounds force target proteins into proximity with E3 ubiquitin ligase and apply the ubiquitin-proteasome system to induce degradation of the target proteins. The technology provides a means of creating medicines for not only conventional targets such as specific enzymes and receptors, but also disease-related proteins for which drug discovery up to this point has been difficult. Through the development of this technology and drug discovery, the University of Tokyo and Eisai aim to provide new treatment options to patients for which treatment options were previously limited.
Media Inquiries
Eisai Co., Ltd.
Public Relations Department
TEL : +81-(0)3-3817-5120
[Notes to editors]?
1. About SNIPER
SNIPER (Specific and Nongenetic IAP-dependent Protein Eraser) is a compound which utilizes the ubiquitin-proteasome system to degrade target proteins. This compound is a “hybrid compound”, and consists of a moiety that binds to the target protein and a moiety that binds to E3 ubiquitin ligase (IAPs) with an appropriate linker. Designing this compound requires advanced medicinal chemistry and cutting-edge structural biology. When the SNIPER compound brings the target protein and E3 ubiquitin ligase (IAPs) into proximity (Step 1 in the chart below), ubiquitin as a protein degradation tag transfers from the E2 ubiquitin conjugating enzyme to the target protein (2) for recognition of the protein by the proteasome and subsequent degradation (3).
Conventional small-molecule inhibitors bind to the active moiety of target enzymes, and express pharmacological activity by inhibiting the activity thereof. On the other hand, because SNIPER exhibits pharmacological properties by target protein degradation as described above, it is not only expected to exhibit different pharmacological activity from small-molecule inhibitors; it is also predicted to target proteins that do not have enzymatic activity. Similar technologies include PROTAC (PROteolysis TArgeting Chimeras) and Degronimid.
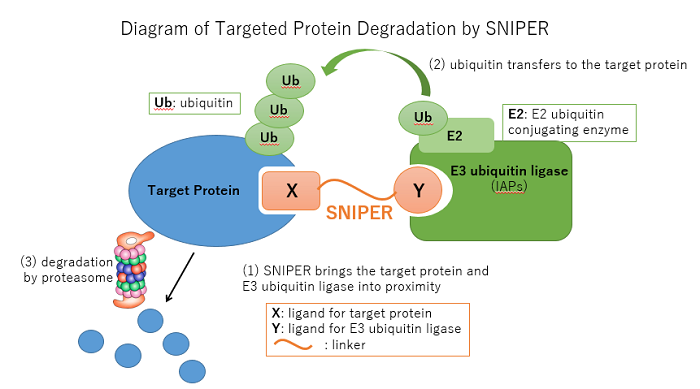
2. About the Ubiquitin-Proteasome System
The ubiquitin-proteasome system is one of the naturally occurring mechanisms for controlling the degradation of unneeded proteins, and is in control of critical movements relating to the preservation of life including cell cycle, transcription regulation, and signal transmission. When ubiquitin as a protein degradation tag connects to unneeded proteins through agents such as the E3 ubiquitin ligase, it marks the protein for recognition by the proteasome for subsequent degradation. In recent years, the relation between the ubiquitin-proteasome system and major human diseases including cancer and neurodegeneration is becoming evident.
Eisai Co., Ltd. (Headquarters: Tokyo, CEO: Haruo Naito, “Eisai”) announced today that it has entered into a joint research agreement with four research organizations (KAN Research Institute, National Center for Global Health and Medicine, Nagasaki University, and Yokohama City University) in Japan concerning the “Development of Therapeutics to Prevent the Aggravation of the Novel Coronavirus Infectious Disease (COVID-19)” (Grant Number: 20fk0108255), which is a research project with Eisai as the representative research organization. This joint research project “Development of Therapeutics for Novel Coronavirus Infectious Disease (COVID-19)” was adopted for the second public call by the Japan Agency for Medical Research and Development (AMED) as part of its operation for promotion of the research and development of innovative treatments for emerging and re-emerging infectious diseases in fiscal year 2020.
In patients with COVID-19 due to the SARS-CoV-2 infection, severe cases such as acute respiratory distress syndrome (ARDS) and subsequent multiple organ failure have been reported. The involvement of the formation and exacerbation of vasculopathy as well as the cytokine storm* in the process of aggravation are assumed. However, at this time, the mechanism of aggravation based on the SARS-CoV-2 infection is not fully understood.
In this collaborative research, a non-clinical animal model of SARS-CoV-2 infection will be constructed. Additionally, TLR (Toll-Like Receptor) 4 antagonist eritoran, discovered by Eisai, and an anti-FKN (fractalkine) antibody E6011, discovered by Eisai’s research subsidiary KAN Research Institute, will be evaluated. In addition, this project will promote biomarker research using clinical samples derived from SARS-CoV-2 infected patients. This collaborative research, aims to elucidate the mechanism of COVID-19 aggravation based on SARS-CoV-2 infection and to create drugs that prevent the aggravation of COVID-19.
In the fight against the expansion of COVID-19, based on the?human health care?(hhc) philosophy, Eisai will continue the development of therapeutics, stable supply of pharmaceuticals, and support activities in each country.
* Cytokine storm: a state of immune runaway, in which the production of cytokines, which play a role in activating the immune response, becomes uncontrollable and cytokines are released in large amounts.
Media Inquiries:
Public Relations Department,
Eisai Co., Ltd.
+81-(0)3-3817-5120
[Notes to editors]
1. About TLR4 and Eritoran (E5564)
TLR(Toll-Like Receptor)s are receptors of the innate immune system, and recognize the specific molecular structure of pathogens. It is considered that TLR initiated activation of the innate immune system plays a critical role in eliminating pathogens, causing an inflammatory reaction or an antiviral response. TLR4, one of the TLRs which constitute a family of various receptors, is activated by endotoxins such as lipopolysaccharide released from bacteria. Eritoran is Eisai’s in-house discovered and developed TLR4 antagonist created by natural product organic synthesis technology. It is a structural analogue of Lipid A, which is an active pharmacophore of endotoxins. It has been previously observed to have well-tolerated safety profile in 14 clinical studies including a large Phase III randomized trial in severe sepsis. Eritoran has been shown to have the effects of suppressing cytokine production and improving systemic condition in a mouse influenza virus infection model1. It is expected to suppress inflammation and aggravation caused by COVID-192,3?by inhibiting the activation of TLR4, which is the most upstream of various cytokine gene expression signaling that causes the cytokine-storm.
Eritoran has been selected as the therapeutic drug candidate in the international trial REMAP-COVID for hospitalized patients with moderate COVID-19.
2. About FKN and E6011
FKN (fractalkine) is a chemokine that has dual functions of cell migration regulation and cell adhesion, which is induced in vascular endothelial cells during inflammation. The FKN receptor (CX3CR1) is mostly expressed in monocytes, macrophages and killer lymphocytes selectively and plays a key role in efficient collection of cells to the inflamed site. It has been suggested that the FKN-CX3CR1 system relates to various chronic inflammatory diseases including inflammatory bowel disease, rheumatoid arthritis, liver disease, central nervous system disease, arteriosclerosis, dermatosis and others. E6011 is the world’s first humanized anti-FKN monoclonal antibody developed by Eisai’s research subsidiary KAN Research Institute, Inc., and has a novel action mechanism inhibiting cell invasion by neutralizing activity of fractalkine (FKN), unlike existing cytokine treatments. Currently, a phase II clinical trial in patients with Crohn’s disease is being conducted by Eisai’s subsidiary for gastrointestinal diseases business EA pharma Co., Ltd. E6011 inhibits tight binding of CD16+ monocytes (cell populations with high CX3CR1 expression), which are important for local inflammatory response, to vascular endothelial cells4. E6011 therefore is expected to suppress the initiation and exacerbation of vasculopathy in COVID-195.
1?KA Shirey et al.,?Nature.?2013?May 23; 497(7450):498-502
2?P Mehta et al.,?The Lancet?2020; 395: 1033-1034
3?C Huang et al.,?The Lancet?2020; 395: 497-506
4?Y Kuboi et al.,?Int Immunol.?2019?Apr 26;31(5):357
5?H Li et al.,?The Lancet?2020; 395: 1517-1520